Values of X-ray wavelengths. What are x-rays - properties and applications of radiation
1. X-ray sources.
2. Bremsstrahlung X-rays.
3. Characteristic x-ray radiation. Moseley's law.
4. Interaction of X-ray radiation with matter. The law of weakening.
5. Physical basis for the use of X-rays in medicine.
6. Basic concepts and formulas.
7. Tasks.
X-ray radiation - electromagnetic waves with a wavelength from 100 to 10 -3 nm. On the scale of electromagnetic waves, X-ray radiation occupies the region between UV radiation and γ -radiation. X-rays (X-rays) were discovered in 1895 by K. Roentgen, who in 1901 became the first Nobel laureate in physics.
32.1. X-ray sources
Natural sources of X-rays are some radioactive isotopes (for example, 55 Fe). Artificial sources of powerful X-rays are x-ray tubes(Fig. 32.1).
Rice. 32.1. X-ray tube device
The X-ray tube is an evacuated glass flask with two electrodes: the anode A and the cathode K, between which a high voltage U (1-500 kV) is created. The cathode is a coil heated by electric current. Electrons emitted by a heated cathode (thermionic emission) are accelerated by an electric field to big speeds (for this you need high voltage) and fall on the anode of the tube. When these electrons interact with the anode material, two types of X-ray radiation arise: brake And characteristic.
The working surface of the anode is located at some angle to the direction of the electron beam in order to create the desired direction of the x-rays.
Approximately 1% of the kinetic energy of electrons is converted into X-rays. The rest of the energy is released as heat. Therefore, the working surface of the anode is made of a refractory material.
32.2. Bremsstrahlung X-ray
An electron moving in some medium loses its speed. This creates a negative acceleration. According to Maxwell's theory, any accelerated the movement of a charged particle is accompanied by electromagnetic radiation. The radiation that occurs when an electron decelerates in the anode material is called bremsstrahlung X-rays.
The properties of bremsstrahlung are determined by the following factors.
1. Radiation is emitted by individual quanta, the energies of which are related to the frequency by the formula (26.10)
![]() where ν is the frequency, λ is the wavelength.
where ν is the frequency, λ is the wavelength.
2. All electrons reaching the anode have the same kinetic energy equal to the work of the electric field between the anode and cathode:
where e is the electron charge, U is the accelerating voltage.
3. The kinetic energy of an electron is partially transferred to the substance and goes to heat it (Q), and is partially spent on the creation of an X-ray quantum:
4. Relationship between Q and hv accidentally.
Due to the last property (4), the quanta generated by various electrons, have various frequencies and wavelengths. Therefore, the bremsstrahlung spectrum is solid. typical view spectral density the X-ray flux (Φ λ = άΦ/άλ) is shown in fig. 32.2.
 Rice. 32.2. Bremsstrahlung spectrum
Rice. 32.2. Bremsstrahlung spectrum
From the side of long waves, the spectrum is limited by a wavelength of 100 nm, which is the boundary of X-ray radiation. From the side of short waves, the spectrum is limited by the wavelength λ min . According to formula (32.2) minimum wavelength corresponds to the case Q = 0 (the kinetic energy of the electron is completely converted into the energy of the quantum):
 Calculations show that the bremsstrahlung flux (Φ) is directly proportional to the square of the voltage U between
Calculations show that the bremsstrahlung flux (Φ) is directly proportional to the square of the voltage U between
anode and cathode, current I in the tube and atomic number Z of the anode substance:
The X-ray bremsstrahlung spectra at various voltages, various cathode temperatures, and various anode materials are shown in Figs. 32.3.
 Rice. 32.3. Bremsstrahlung spectrum (Φ λ):
Rice. 32.3. Bremsstrahlung spectrum (Φ λ):
a - at different voltages U in the tube; b - at different temperatures T
cathode; c - with different anode substances differing in parameter Z
With an increase in the anode voltage, the value λmin shifts towards shorter wavelengths. At the same time, the height of the spectral curve also increases (Fig. 32.3, A).
As the cathode temperature increases, the electron emission increases. Correspondingly, the current I in the tube also increases. The height of the spectral curve increases, but the spectral composition of the radiation does not change (Fig. 32.3, b).
When the anode material changes, the height of the spectral curve changes in proportion to the atomic number Z (Fig. 32.3, c).
32.3. Characteristic x-ray radiation. Moseley's law
When cathode electrons interact with anode atoms, along with X-ray bremsstrahlung, X-ray radiation arises, the spectrum of which consists of individual lines. This radiation
has the following origin. Some cathodic electrons penetrate deep into the atom and knock electrons out of it. inner shells. The vacancies thus formed are filled with electrons with upper shells, resulting in the emission of radiation quanta. This radiation contains a discrete set of frequencies determined by the anode material and is called characteristic radiation. The full spectrum of an x-ray tube is a superposition of the characteristic spectrum on the bremsstrahlung spectrum (Fig. 32.4).
 Rice. 32.4. X-ray tube emission spectrum
Rice. 32.4. X-ray tube emission spectrum
The existence of characteristic X-ray spectra has been discovered using X-ray tubes. Later it was found that such spectra arise during any ionization of the inner orbits of chemical elements. Having studied the characteristic spectra of various chemical elements, G. Moseley (1913) established the following law, which bears his name.
The square root of the frequency of the characteristic radiation is a linear function of the ordinal number of the element:
where ν is the frequency of the spectral line, Z is the atomic number of the emitting element, A, B are constants.
Moseley's law makes it possible to determine the atomic number of a chemical element from the observed spectrum of characteristic radiation. This played a big role in the placement of elements in the periodic system.
32.4. Interaction of X-ray radiation with matter. law of weakening
There are two main types of interaction of X-ray radiation with matter: scattering and photoelectric effect. When scattered, the direction of motion of a photon changes. In the photoelectric effect, a photon absorbed.
1. Coherent (elastic) scattering occurs when the energy of an X-ray photon is insufficient for the internal ionization of an atom (knocking out an electron from one of the inner shells). In this case, the direction of motion of the photon changes, and its energy and wavelength do not change (therefore, this scattering is called elastic).
 2. Incoherent (Compton) scattering occurs when the photon energy is much greater than the internal ionization energy A u: hv >> A u.
2. Incoherent (Compton) scattering occurs when the photon energy is much greater than the internal ionization energy A u: hv >> A u.
In this case, the electron breaks away from the atom and acquires some kinetic energy E k. The direction of the photon during Compton scattering changes, and its energy decreases:
Compton scattering is associated with the ionization of the atoms of matter.
 3. photoelectric effect occurs when the photon energy hv is sufficient to ionize the atom: hv > A u. At the same time, the X-ray quantum absorbed and its energy is spent on the ionization of the atom and the communication of kinetic energy to the ejected electron E k \u003d hv - AI.
3. photoelectric effect occurs when the photon energy hv is sufficient to ionize the atom: hv > A u. At the same time, the X-ray quantum absorbed and its energy is spent on the ionization of the atom and the communication of kinetic energy to the ejected electron E k \u003d hv - AI.
 Compton scattering and the photoelectric effect are accompanied by characteristic X-ray radiation, since after the knocking out of internal electrons, the vacancies are filled with electrons from the outer shells.
Compton scattering and the photoelectric effect are accompanied by characteristic X-ray radiation, since after the knocking out of internal electrons, the vacancies are filled with electrons from the outer shells.
X-ray luminescence. In some substances, electrons and quanta of Compton scattering, as well as photoelectric effect electrons, cause excitation of molecules, which is accompanied by radiative transitions to the ground state. This produces a glow called X-ray luminescence. The luminescence of barium platinum-cyanogen allowed X-rays to be discovered by Roentgen.
law of weakening
The scattering of X-rays and the photoelectric effect lead to the fact that as the X-ray radiation penetrates deep into the primary beam of radiation is weakened (Fig. 32.5). The easing is exponential:
 The value of μ depends on the absorbing material and the radiation spectrum. For practical calculations, as a characteristic of the weakened
The value of μ depends on the absorbing material and the radiation spectrum. For practical calculations, as a characteristic of the weakened
 Rice. 32.5. Attenuation of the X-ray flux in the direction of the incident rays
Rice. 32.5. Attenuation of the X-ray flux in the direction of the incident rays
 Where λ
- wavelength; Z is the atomic number of the element; k is some constant.
Where λ
- wavelength; Z is the atomic number of the element; k is some constant.
32.5. Physical bases of use
x-ray radiation in medicine
In medicine, X-rays are used for diagnostic and therapeutic purposes.
X-ray diagnostics- Methods for obtaining images of internal organs using x-rays.
The physical basis of these methods is the law of X-ray attenuation in matter (32.10). Cross-sectional uniform X-ray flux after passing through inhomogeneous tissue will become inhomogeneous. This inhomogeneity can be recorded on photographic film, a fluorescent screen, or using a matrix photodetector. For example, the mass weakening coefficients of bone tissue - Ca 3 (PO 4) 2 - and soft tissues - mainly H 2 O - differ by 68 times (μ m bone /μ m water = 68). Bone density is also higher than soft tissue density. Therefore, an x-ray image produces a light image of the bone against a darker background of soft tissues.
If the organ under study and the tissues surrounding it have similar attenuation coefficients, then special contrast agents. So, for example, during fluoroscopy of the stomach, the subject takes a mushy mass of barium sulfate (BaSO 4), in which the mass attenuation coefficient is 354 times greater than that of soft tissues.
For diagnostics, X-ray radiation with a photon energy of 60-120 keV is used. In medical practice, the following methods of X-ray diagnostics are used.
1. X-ray. The image is formed on a fluorescent screen. The image brightness is low and can only be viewed in a darkened room. The physician must be protected from exposure.
The advantage of fluoroscopy is that it is carried out in real time. The disadvantage is a large radiation load on the patient and the doctor (compared to other methods).
The modern version of fluoroscopy - X-ray television - uses X-ray image intensifiers. The amplifier perceives the weak glow of the X-ray screen, amplifies it and transmits it to the TV screen. As a result, the radiation load on the doctor has sharply decreased, the brightness of the image has increased, and it has become possible to record the results of the examination on video.
2. Radiography. The image is formed on a special film that is sensitive to x-rays. Pictures are taken in two mutually perpendicular projections (direct and lateral). The image becomes visible after photo processing. The finished dried image is viewed in transmitted light.
At the same time, details are satisfactorily visible, the contrast of which differs by 1-2%.
In some cases, before the examination, the patient is given a special contrast agent. For example, an iodine-containing solution (intravenously) in the study of the kidneys and urinary tract.
The advantages of radiography are high resolution, short exposure time and almost complete safety for the doctor. The disadvantages include the static image (the object cannot be traced in dynamics).
3. Fluorography. In this examination, the image obtained on the screen is photographed on a sensitive small format film. Fluorography is widely used in the mass survey of the population. If pathological changes are found on the fluorogram, then the patient is prescribed a more detailed examination.
4. Electroroentgenography. This type of examination differs from conventional radiography in the way the image is captured. Use instead of film selenium plate, electrified by X-rays. The result is a latent image of electrical charges that can be made visible and transferred to paper.
5. Angiography. This method is used in the examination of blood vessels. A contrast agent is injected into the vein through a catheter, after which a powerful x-ray machine takes a series of images following each other in a fraction of a second. Figure 32.6 shows an angiogram in the region of the carotid artery.
6. X-ray computed tomography. This type of X-ray examination allows you to get an image of a flat section of the body with a thickness of several mm. In this case, the given section is repeatedly illuminated at different angles with the fixation of each individual image in the computer's memory. Then
 Rice. 32.6. Angiogram showing a narrowing in the canal of the carotid artery
Rice. 32.6. Angiogram showing a narrowing in the canal of the carotid artery
 Rice. 32.7. Scanning scheme of tomography (a); tomogram of the head in cross section at eye level (b).
Rice. 32.7. Scanning scheme of tomography (a); tomogram of the head in cross section at eye level (b).
computer reconstruction is carried out, the result of which is the image of the scanned layer (Fig. 32.7).
Computed tomography makes it possible to distinguish elements with a density difference between them up to 1%. Conventional radiography allows you to capture a minimum difference in density between adjacent areas of 10-20%.
X-ray therapy - the use of x-rays to destroy malignant tumors.
The biological effect of radiation is to disrupt the vital activity of especially rapidly multiplying cells. Very hard X-rays (with a photon energy of approximately 10 MeV) are used to destroy cancer cells deep within the body. To reduce damage to healthy surrounding tissues, the beam rotates around the patient in such a way that only the damaged area remains under its influence at all times.
32.6. Basic concepts and formulas
 Table continuation
Table continuation
 End of table
End of table
 32.7. Tasks
32.7. Tasks
1. Why does an electron beam in medical X-ray tubes strike one point of the anticathode, and does not fall on it in a wide beam?
Answer: to obtain a point source of x-rays, giving a sharp outline of translucent objects on the screen.
2. Find the boundary of bremsstrahlung X-rays (frequency and wavelength) for voltages U 1 = 2 kV and U 2 = 20 kV.
 4.
Lead screens are used to protect against x-rays. The linear absorption of X-rays in lead is 52 cm -1 . What should be the thickness of the shielding layer of lead in order for it to reduce the X-ray intensity by 30 times?
4.
Lead screens are used to protect against x-rays. The linear absorption of X-rays in lead is 52 cm -1 . What should be the thickness of the shielding layer of lead in order for it to reduce the X-ray intensity by 30 times?
 5.
Find the X-ray tube radiation flux at U = 50 kV, I = 1 mA. The anode is made of tungsten (Z = 74). Find the efficiency of the tube.
5.
Find the X-ray tube radiation flux at U = 50 kV, I = 1 mA. The anode is made of tungsten (Z = 74). Find the efficiency of the tube.
 6.
For X-ray diagnostics of soft tissues, contrast agents are used. For example, the stomach and intestines are filled with a mass of barium sulfate (BaSO 4 ). Compare the mass attenuation coefficients of barium sulfate and soft tissues (water).
6.
For X-ray diagnostics of soft tissues, contrast agents are used. For example, the stomach and intestines are filled with a mass of barium sulfate (BaSO 4 ). Compare the mass attenuation coefficients of barium sulfate and soft tissues (water).
 7. What will give a thicker shadow on the X-ray screen: aluminum (Z = 13, ρ = 2.7 g/cm 3) or the same layer of copper (Z = 29, ρ = 8.9 g/cm 3)?
7. What will give a thicker shadow on the X-ray screen: aluminum (Z = 13, ρ = 2.7 g/cm 3) or the same layer of copper (Z = 29, ρ = 8.9 g/cm 3)?
 8.
How many times is the thickness of the aluminum layer greater than the thickness of the copper layer, if the layers attenuate x-rays in the same way?
8.
How many times is the thickness of the aluminum layer greater than the thickness of the copper layer, if the layers attenuate x-rays in the same way?
1. Bremsstrahlung and characteristic x-rays,
basic properties and characteristics.
In 1895, the German scientist Roentgen first discovered the glow of a fluorescent screen, which was caused by radiation invisible to the eye coming from a portion of the gas discharge tube glass located opposite the cathode. This type of radiation had the ability to pass through substances impenetrable to visible light. Roentgen called them X-rays and established the basic properties that make it possible to use them in various branches of science and technology, including medicine.
X-ray is called radiation with a wavelength of 80-10 -5 nm. Long-wave X-ray radiation overlaps short-wave UV radiation, short-wave overlaps with long-wave g-radiation. In medicine, X-ray radiation with a wavelength of 10 to 0.005 nm is used, which corresponds to a photon energy of 10 2 EV to 0.5 MeV. X-ray radiation is invisible to the eye, therefore, all observations with it are made using fluorescent screens or photographic films, since it causes x-ray luminescence and has a photochemical effect. It is characteristic that the majority of bodies that are impenetrable to optical radiation are largely transparent to X-ray radiation, which has properties common to electromagnetic waves. However, due to the smallness of the wavelength, some properties are difficult to detect. Therefore, the wave nature of radiation was established much later than their discovery.
According to the method of excitation, X-ray radiation is divided into bremsstrahlung and characteristic radiation.
Bremsstrahlung X-rays are due to the deceleration of fast moving electrons by the electric field of the atom (nucleus and electrons) of the substance through which they fly. The mechanism of this radiation can be explained by the fact that any moving charge is a current around which a magnetic field is created, the induction (B) of which depends on the speed of the electron. When braking, the magnetic induction decreases and, in accordance with Maxwell's theory, an electromagnetic wave appears.
When electrons decelerate, only part of the energy goes to create an X-ray photon, the other part is spent on heating the anode. The frequency (wavelength) of a photon depends on the initial kinetic energy of the electron and the intensity of its deceleration. Moreover, even if the initial kinetic energy is the same, then the deceleration conditions in the substance will be different, therefore, the emitted photons will have the most diverse energy, and, consequently, the wavelength, i.e. the X-ray spectrum will be continuous. Figure 1 shows the bremsstrahlung spectrum at different voltages U 1
 .
.
If U is expressed in kilovolts and the ratio between other quantities is taken into account, then the formula looks like: l k \u003d 1.24 / U (nm) or l k \u003d 1.24 / U (Å) (1Å \u003d 10 -10 m).
From the graphs above, it can be established that the wavelength l m, which accounts for the maximum radiation energy, is in constant relation to the limiting wavelength l k:
![]() .
.
The wavelength characterizes the energy of a photon, on which the penetrating power of radiation depends when it interacts with matter.
Short-wavelength X-rays usually have a high penetrating power and are called hard, while long-wavelength X-rays are called soft. As can be seen from the above formula, the wavelength at which the maximum radiation energy falls is inversely proportional to the voltage between the anode and cathode of the tube. Increasing the voltage at the anode of the x-ray tube, change the spectral composition of the radiation and increase its hardness.
When the filament voltage changes (the filament temperature of the cathode changes), the number of electrons emitted by the cathode per unit time changes, or, accordingly, the current strength in the tube anode circuit. In this case, the radiation power changes in proportion to the first power of the current. The spectral composition of the radiation will not change.
The total flux (power) of radiation, the distribution of energy over wavelengths, and also the boundary of the spectrum on the side of short wavelengths depend on the following three factors: voltage U, which accelerates electrons and is applied between the anode and cathode of the tube; the number of electrons involved in the formation of radiation, i.e. tube filament current; atomic number Z of the anode material, in which the electron deceleration occurs.
The bremsstrahlung flux is calculated by the formula: , where ![]() ,
,
Z-serial number of an atom of a substance (atomic number).
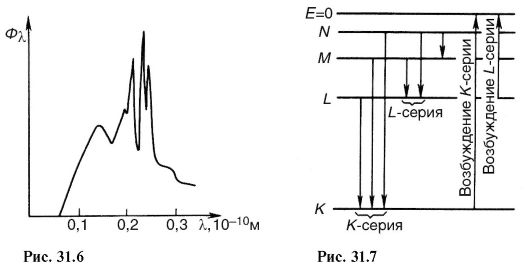
By increasing the voltage on the x-ray tube, one can notice the appearance of separate lines (line spectrum) against the background of continuous bremsstrahlung radiation, which corresponds to the characteristic x-ray radiation. It arises during the transition of electrons between the inner shells of atoms in a substance (shells K, L, M). The line character of the characteristic radiation spectrum arises due to the fact that accelerated electrons penetrate deep into the atoms and knock out electrons from their inner layers outside the atom. Electrons (Fig. 2) from the upper layers pass to free places, as a result of which X-ray photons are emitted with a frequency corresponding to the difference in the transition energy levels. The lines in the spectrum of characteristic radiation are combined into series corresponding to transitions of electrons with a higher level at the level of K, L, M.
The external action, as a result of which the electron is knocked out of the inner layers, must be strong enough. In contrast to optical spectra, the characteristic x-ray spectra of different atoms are of the same type. The uniformity of these spectra is due to the fact that the inner layers of different atoms are the same and differ only energetically, because the force effect from the side of the nucleus increases as the ordinal number of the element increases. This leads to the fact that the characteristic spectra shift towards higher frequencies with increasing nuclear charge. This relationship is known as Moseley's law: ![]() , where A and B are constants; Z-order number of the element.
, where A and B are constants; Z-order number of the element.
There is another difference between X-ray and optical spectra. The characteristic spectrum of an atom does not depend on the chemical compound in which the atom is included. So, for example, the X-ray spectrum of the oxygen atom is the same for O, O 2 , H 2 O, while the optical spectra of these compounds are significantly different. This feature of the x-ray spectra of atoms served as the basis for the name "characteristic".
Characteristic radiation occurs whenever there are free places in the inner layers of an atom, regardless of the reasons that caused it. For example, it accompanies one of the types of radioactive decay, which consists in the capture of an electron from the inner layer by the nucleus.
2. The device of x-ray tubes and protozoa
x-ray machine.
The most common source of X-ray radiation is an X-ray tube - a two-electrode vacuum device (Fig. 3). It is a glass container (p = 10 -6 - 10 -7 mm Hg) with two electrodes - anode A and cathode K, between which a high voltage is created. The heated cathode (K) emits electrons. Anode A is often referred to as the anticathode. It has an inclined surface in order to direct the resulting X-ray radiation at an angle to the axis of the tube. The anode is made of a metal with good thermal conductivity (copper) to remove the heat generated by the impact of electrons. At the beveled end of the anode there is a plate Z made of refractory metal (tungsten) with a high atomic number, called the anode mirror. In some cases, the anode is specially cooled with water or oil. For diagnostic tubes, the pinpointness of the X-ray source is important, which can be achieved by focusing the electrons in one place of the anode. Therefore, constructively, two opposite tasks have to be taken into account: on the one hand, electrons must fall on one place of the anode, on the other hand, in order to prevent overheating, it is desirable to distribute electrons over different parts of the anode. For this reason, some X-ray tubes are manufactured with a rotating anode.
In a tube of any design, electrons accelerated by the voltage between the anode and the cathode fall on the anode mirror and penetrate deep into the substance, interact with atoms and are decelerated by the field of atoms. This produces bremsstrahlung X-rays. Simultaneously with the bremsstrahlung, a small amount (several percent) of characteristic radiation is formed. Only 1-2% of the electrons that hit the anode cause bremsstrahlung, and the rest cause a thermal effect. For the concentration of electrons, the cathode has a guide cap. The part of the tungsten mirror on which the main electron flow falls is called the focus of the tube. The width of the radiation beam depends on its area (focus sharpness).
To power the tube, two sources are required: a high voltage source for the anode circuit and a low voltage source (6-8 V) to power the filament circuit. Both sources must be independently regulated. By changing the anode voltage, the hardness of the X-ray radiation is regulated, and by changing the incandescence, the current of the output circuit and, accordingly, the radiation power.
Schematic diagram of the simplest X-ray machine is shown in Fig.4. The circuit has two high voltage transformers Tr.1 and Tr.2 for powering the filament. The high voltage on the tube is regulated by an autotransformer Tr.3 connected to the primary winding of the transformer Tr.1. Switch K regulates the number of turns of the autotransformer winding. In this regard, the voltage of the secondary winding of the transformer, supplied to the anode of the tube, also changes, i.e. hardness is adjustable.
The filament current of the tube is regulated by a rheostat R, included in the primary circuit of the transformer Tr.2. The anode circuit current is measured with a milliammeter. The voltage applied to the electrodes of the tube is measured with a kV kilovoltmeter, or the voltage in the anode circuit can be judged by the position of switch K. The filament current, regulated by the rheostat, is measured with an ammeter A. In the scheme under consideration, the x-ray tube simultaneously rectifies a high alternating voltage.
It is easy to see that such a tube radiates only in one half-cycle of alternating current. Therefore, its power will be small. In order to increase the radiated power, many devices use high-voltage full-wave X-ray rectifiers. For this purpose, 4 special kenotrons are used, which are connected in a bridge circuit. An x-ray tube is included in one diagonal of the bridge.
3. Interaction of X-ray radiation with matter
(coherent scattering, incoherent scattering, photoelectric effect).
When X-rays fall on a body, it is reflected from it in a small amount, but mostly passes deep into. In the mass of the body, radiation is partially absorbed, partially scattered, and partially passes through. Passing through the body, X-ray photons interact mainly with the electrons of the atoms and molecules of the substance. Registration and use of X-ray radiation, as well as its impact on biological objects, is determined by the primary processes of interaction of an X-ray photon with electrons. Three main processes take place depending on the ratio of photon energy E and ionization energy AI.
A) coherent scattering.
Scattering of long-wavelength X-rays occurs mainly without changing the wavelength, and it is called coherent. The interaction of a photon with the electrons of the inner shells, tightly bound to the nucleus, only changes its direction, without changing its energy, and hence the wavelength (Fig. 5).
Coherent scattering occurs if the photon energy is less than the ionization energy: E = hn<А И. Так как энергия фотона и энергия атома не изменяется, то когерентное рассеяние не вызывает биологического действия. Однако при создании защиты от рентгеновского излучения следует учитывать возможность изменения направления первичного пучка.
b) Incoherent scattering (Compton effect).
In 1922, A. Compton, observing the scattering of hard X-rays, discovered a decrease in the penetrating power of the scattered beam compared to the incident beam. The scattering of X-rays with changing wavelength is called the Compton effect. It occurs when a photon of any energy interacts with the electrons of the outer shells of atoms weakly bound to the nucleus (Fig. 6). An electron is detached from an atom (such electrons are called recoil electrons). The energy of the photon decreases (the wavelength increases accordingly), and the direction of its movement also changes. The Compton effect occurs if the X-ray photon energy is greater than the ionization energy: , . In this case, recoil electrons with kinetic energy E K appear. Atoms and molecules become ions. If E K is significant, then electrons can ionize neighboring atoms by collision, forming new (secondary) electrons.
V) Photoelectric effect.
If the energy of a photon hn is sufficient to detach an electron, then when interacting with an atom, the photon is absorbed, and the electron is detached from it. This phenomenon is called the photoelectric effect. The atom is ionized (photoinization). In this case, the electron acquires kinetic energy and, if the latter  is significant, then it can ionize neighboring atoms by collision, forming new (secondary) electrons. If the photon energy is insufficient for ionization, then the photoelectric effect can manifest itself in the excitation of an atom or molecule. In some substances, this leads to the subsequent emission of photons in the visible radiation region (X-ray luminescence), and in tissues, to the activation of molecules and photochemical reactions.
is significant, then it can ionize neighboring atoms by collision, forming new (secondary) electrons. If the photon energy is insufficient for ionization, then the photoelectric effect can manifest itself in the excitation of an atom or molecule. In some substances, this leads to the subsequent emission of photons in the visible radiation region (X-ray luminescence), and in tissues, to the activation of molecules and photochemical reactions.
The photoelectric effect is typical for photons with an energy of the order of 0.5-1 MeV.
The three main interaction processes discussed above are primary, they lead to subsequent secondary, tertiary, etc. phenomena. When X-ray radiation enters a substance, a number of processes can occur before the energy of an X-ray photon is converted into the energy of thermal motion.
As a result of the above processes, the primary X-ray flux is weakened. This process obeys Bouguer's law. We write it in the form: Ф =Ф 0 e - mx, where m is a linear attenuation coefficient that depends on the nature of the substance (mainly on density and atomic number) and on the radiation wavelength (photon energy). It can be represented as consisting of three terms corresponding to coherent scattering, incoherent scattering, and the photoelectric effect: ![]() .
.
Since the linear absorption coefficient depends on the density of the substance, it is preferable to use the mass attenuation coefficient, which is equal to the ratio of the linear attenuation coefficient to the density of the absorber and does not depend on the density of the substance. The dependence of the X-ray flux (intensity) on the thickness of the absorbing filter is shown in Fig. 7 for H 2 O, Al, and Cu. Calculations show that a layer of water 36 mm thick, aluminum 15 mm and copper 1.6 mm reduce the X-ray intensity by 2 times. This thickness is called the half layer thickness d. If a substance attenuates X-ray radiation by half, then ![]() , Then
, Then ![]() , or ,
, or , ![]() ; ; . Knowing the thickness of the half layer, you can always determine m. Dimension .
; ; . Knowing the thickness of the half layer, you can always determine m. Dimension .
4. The use of x-rays in medicine
(fluoroscopy, radiography, X-ray tomography, fluorography, radiotherapy).
One of the most common applications of X-rays in medicine is the transillumination of internal organs for diagnostic purposes - X-ray diagnostics.
For diagnostics, photons with an energy of 60-120 keV are used. In this case, the mass absorption coefficient is determined mainly by the photoelectric effect. Its value is proportional to l 3 (in which the large penetrating power of hard radiation is manifested) and proportional to the third power of the number of atoms of the substance - absorber: , where K is the coefficient of proportionality.
The human body consists of tissues and organs that have different absorbing capacity in relation to X-rays. Therefore, when it is illuminated with X-rays, a non-uniform shadow image is obtained on the screen, which gives a picture of the location of internal organs and tissues. The densest radiation-absorbing tissues (heart, large vessels, bones) are seen as dark, while the less absorbing tissues (lungs) are seen as light.
In many cases, it is possible to judge their normal or pathological state. X-ray diagnostics uses two main methods: fluoroscopy (transmission) and radiography (image). If the organ under study and the tissues surrounding it approximately equally absorb the X-ray flux, then special contrast agents are used. So, for example, on the eve of an X-ray examination of the stomach or intestines, a mushy mass of barium sulfate is given, in which case one can see their shadow image. In fluoroscopy and radiography, an x-ray image is a summary image of the entire thickness of the object through which the x-rays pass. The most clearly defined are those details that are closer to the screen or film, and the distant ones become fuzzy and blurry. If in some organ there is a pathologically altered area, for example, the destruction of lung tissue inside an extensive focus of inflammation, then in some cases this area on the x-ray in the amount of shadows can be “lost”. To make it visible, a special method is used - tomography (layered recording), which allows you to take pictures of individual layers of the area under study. This kind of layer-by-layer tomograms is obtained using a special apparatus called a tomograph, in which the X-ray tube (RT) and film (Fp) are periodically, jointly, in antiphase moved relative to the study area. In this case, X-rays at any position of the RT will pass through the same point of the object (changed area), which is the center relative to which the RT and FP periodically move. The shadow image of the area will be captured on film. By changing the position of the “swing center”, it is possible to obtain layered images of the object. Using a thin beam of X-rays, a special screen (instead of Fp) consisting of semiconductor detectors of ionizing radiation, it is possible to process the image during tomography using a computer. This modern variant of tomography is called computed tomography. Tomography is widely used in the study of the lungs, kidneys, gallbladder, stomach, bones, etc.
The brightness of the image on the screen and the exposure time on the film depends on the intensity of the X-ray radiation. When using it for diagnostics, the intensity cannot be high, so as not to cause an undesirable biological effect. Therefore, there are a number of technical devices that improve the brightness of the image at low X-ray intensities. One of these devices is an image intensifier tube.
Another example is fluorography, in which an image is obtained on a sensitive small-format film from a large X-ray luminescent screen. When shooting, a lens of large aperture is used, the finished pictures are examined on a special magnifier.
Fluorography combines a great ability to detect latent diseases (diseases of the chest, gastrointestinal tract, paranasal sinuses, etc.) with a significant throughput, and therefore is a very effective method of mass (in-line) research.
Since photographing an x-ray image during fluorography is performed using photographic optics, the image on the fluorogram is reduced compared to the x-ray. In this regard, the resolution of the fluorogram (i.e., the visibility of small details) is less than that of a conventional radiograph, however, it is greater than with fluoroscopy.
A device was designed - a tomofluorograph, which makes it possible to obtain fluorograms of body parts and individual organs at a given depth - the so-called layered images (sections) - tomofluorograms.
X-ray radiation is also used for therapeutic purposes (X-ray therapy). The biological effect of radiation is to disrupt the vital activity of cells, especially rapidly developing ones. In this regard, X-ray therapy is used to influence malignant tumors. It is possible to choose a dose of radiation sufficient for the complete destruction of the tumor with relatively minor damage to the surrounding healthy tissues, which are restored due to subsequent regeneration.
The action of X-ray radiation on a substance is determined by the primary processes of interaction of an X-ray photon with the electrons of atoms and molecules of the substance.
3. X-ray computed tomography.
The method of X-ray computed tomography is based on the reconstruction of an image of a certain section (section) of the patient's body by recording a large number of X-ray projections of this section, made at different angles (Fig. 5). Information from the sensors that register these projections enters the computer, which, according to a special program, calculates distribution sample density in the investigated section and displays it on the display screen. The image of the section of the patient's body obtained in this way is characterized by excellent clarity and high information content. The program allows you to increase image contrast tens or even hundreds of times. This expands the diagnostic capabilities of the method.
Rice. Fig. 5. Scheme of X-ray transillumination of a section of the organ under study (point 1 and point 2 - two consecutive positions of the X-ray source)
4. With fluorography an image from a large screen is recorded on a sensitive small-format film (Fig. 6). During analysis, the images are examined on a special magnifier.
This method is used for mass survey of the population. In this case, the radiation load on the patient is much less than in conventional fluoroscopy.
X-ray therapy- the use of X-rays to destroy malignant tumors.
The biological effect of radiation is to disrupt the vital activity of rapidly multiplying tumor cells. In this case, the energy of R - photons is 150-200 keV.
Visiographs (devices with digital X-ray image processing) in modern dentistry
In dentistry, X-ray examination is the main diagnostic method. However, a number of traditional organizational and technical features of X-ray diagnostics make it not quite comfortable for both the patient and dental clinics. This is, first of all, the need for the patient to come into contact with ionizing radiation, which often creates a significant radiation load on the body, it is also the need for a photoprocess, and, consequently, the need for photoreagents, including toxic ones. This is, finally, a bulky archive, heavy folders and envelopes with x-ray films.
In addition, the current level of development of dentistry makes the subjective assessment of radiographs by the human eye insufficient. As it turned out, of the variety of shades of gray contained in the x-ray image, the eye perceives only 64.
Obviously, in order to obtain a clear and detailed image of the hard tissues of the dento-jaw system with minimal radiation exposure, other solutions are needed. Today, the search has led to the creation of so-called radiographic systems, videographers - digital radiography systems (1987, Trophy company).
Without technical details, the principle of operation of such systems is as follows. X-ray radiation enters through the object not on a photosensitive film, but on a special intraoral sensor (special electronic matrix). The corresponding signal from the matrix is transmitted to a digitizing device (analog-to-digital converter, ADC) that converts it into digital form and is connected to the computer. Special software builds an x-ray image on the computer screen and allows you to process it, save it on a hard or flexible storage medium (hard drive, disk), print it as a picture as a file.
In a digital system, an x-ray image is a collection of dots, which correspond to different shades of gray. The information display optimization provided by the program makes it possible to obtain an optimal frame in terms of brightness and contrast at a relatively low radiation dose.
In modern systems, created, for example, by Trophy (France) or Schick (USA), 4096 shades of gray are used when forming a frame, the exposure time depends on the object of study and, on average, is hundredths - tenths of a second, a decrease in radiation exposure in relation to to film - up to 90% for intraoral systems, up to 70% for panoramic videographers.
When processing images, videographers allow:
1. Get positive and negative images, false color images, relief images.
2. Increase the contrast and enlarge the part of the image of interest.
3. Evaluate the change in the density of dental tissues and bone structures, control the uniformity of filling the canals.
4. In endodontics, determine the length of the canal of any curvature, and in surgery, select the size of the implant with an accuracy of 0.1 mm.
The unique Caries detector system with elements of artificial intelligence during the analysis of the image allows you to detect caries in the stain stage, root caries and hidden caries.
Solve problems:
1. How many times is the maximum energy of an X-ray bremsstrahlung quantum that occurs at a tube voltage of 80 kV greater than the energy of a photon corresponding to green light with a wavelength of 500 nm?
2. Determine the minimum wavelength in the spectrum of radiation resulting from deceleration on the target of electrons accelerated in the betatron to an energy of 60 MeV.
3. The layer of half attenuation of monochromatic X-ray radiation in some substance is 10 mm. Find the attenuation of this radiation in the given substance.
[*] Φ l - the ratio of energy emitted in a narrow range of wavelengths for 1s. to the width of this interval
* "F" in formula (4) refers to the entire range of radiated wavelengths and is often referred to as "Integral Energy Flux".
X-rays are electromagnetic waves with a wavelength of approximately 80 to 10 -5 nm. The longest-wavelength X-ray radiation is covered by short-wavelength ultraviolet, the short-wavelength - by long-wavelength γ-radiation. According to the method of excitation, X-ray radiation is divided into bremsstrahlung and characteristic.
31.1. DEVICE OF X-RAY TUBE. Bremsstrahlung X-RAY
The most common source of x-rays is the x-ray tube, which is a two-electrode vacuum device (Fig. 31.1). Heated cathode 1 emits electrons 4. Anode 2, often referred to as the anticathode, has an inclined surface in order to direct the resulting X-rays 3 at an angle to the axis of the tube. The anode is made of a highly heat-conducting material to remove the heat generated by the impact of electrons. The anode surface is made of refractory materials having a large atomic number in the periodic table, such as tungsten. In some cases, the anode is specially cooled with water or oil.
For diagnostic tubes, the pinpointness of the X-ray source is important, which can be achieved by focusing electrons in one place of the anticathode. Therefore, constructively, two opposite tasks have to be taken into account: on the one hand, electrons must fall on one place of the anode, on the other hand, in order to prevent overheating, it is desirable to distribute electrons over different parts of the anode. As one of the interesting technical solutions is an X-ray tube with a rotating anode (Fig. 31.2).
As a result of deceleration of an electron (or other charged particle) by the electrostatic field of the atomic nucleus and atomic electrons of the substance of the anticathode, a bremsstrahlung radiation.
Its mechanism can be explained as follows. A moving electric charge is associated with a magnetic field, the induction of which depends on the speed of the electron. When braking, the magnetic
induction and, in accordance with Maxwell's theory, an electromagnetic wave appears.
When electrons decelerate, only part of the energy goes to create an X-ray photon, the other part is spent on heating the anode. Since the ratio between these parts is random, when a large number of electrons decelerate, a continuous spectrum of X-ray radiation is formed. In this regard, bremsstrahlung is also called continuous. On fig. 31.3 shows the dependence of the X-ray flux on the wavelength λ (spectra) at different voltages in the X-ray tube: U 1< U 2 < U 3 .
In each of the spectra, the shortest wavelength bremsstrahlung λ ηίη arises when the energy acquired by an electron in an accelerating field is completely converted into the energy of a photon:

Note that on the basis of (31.2) one of the most accurate methods for the experimental determination of Planck's constant has been developed.
Short-wavelength X-rays usually have a greater penetrating power than long-wavelength ones and are called hard, and longwave soft.
By increasing the voltage on the X-ray tube, the spectral composition of the radiation is changed, as can be seen from Fig. 31.3 and formulas (31.3), and increase the rigidity.
If the cathode filament temperature is increased, then the electron emission and the current in the tube will increase. This will increase the number of X-ray photons emitted every second. Its spectral composition will not change. On fig. 31.4 shows the X-ray bremsstrahlung spectra at the same voltage, but at different cathode filament currents: / n1< / н2 .
The X-ray flux is calculated by the formula:

Where U And I- voltage and current in the x-ray tube; Z- serial number of an atom of the anode substance; k- coefficient of proportionality. Spectra obtained from different anticathodes at the same U and I H are shown in fig. 31.5.

31.2. CHARACTERISTIC X-RAY RADIATION. ATOMIC X-RAY SPECTRA
By increasing the voltage on the X-ray tube, one can notice the appearance of a line, which corresponds to
characteristic x-rays(Fig. 31.6). It arises due to the fact that accelerated electrons penetrate deep into the atom and knock out electrons from the inner layers. Electrons from upper levels move to free places (Fig. 31.7), as a result, photons of characteristic radiation are emitted. As can be seen from the figure, the characteristic X-ray radiation consists of series K, L, M etc., the name of which served to designate the electronic layers. Since the emission of the K-series frees up space in the higher layers, the lines of other series are simultaneously emitted.
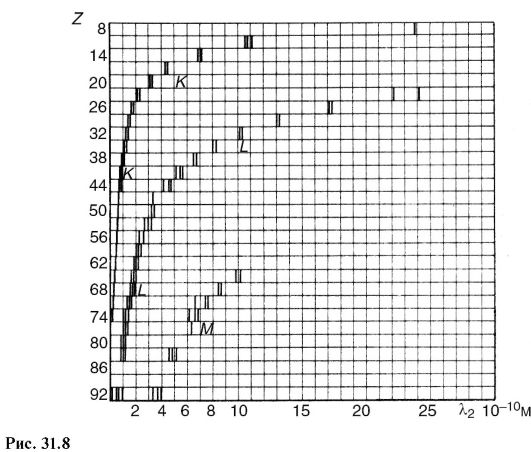
In contrast to optical spectra, the characteristic x-ray spectra of different atoms are of the same type. On fig. 31.8 shows the spectra of various elements. The uniformity of these spectra is due to the fact that the inner layers of different atoms are the same and differ only energetically, since the force effect from the nucleus increases as the element's atomic number increases. This circumstance leads to the fact that the characteristic spectra shift towards higher frequencies with increasing nuclear charge. This pattern is visible from Fig. 31.8 and known as Moseley's law:
Where v- spectral line frequency; Z- atomic number of the emitting element; A And IN- permanent.
There is another difference between optical and x-ray spectra.
The characteristic X-ray spectrum of an atom does not depend on the chemical compound in which this atom is included. For example, the X-ray spectrum of the oxygen atom is the same for O, O 2 and H 2 O, while the optical spectra of these compounds are significantly different. This feature of the x-ray spectrum of the atom was the basis for the name characteristic.
Characteristic radiation always occurs when there is free space in the inner layers of an atom, regardless of the reason that caused it. So, for example, characteristic radiation accompanies one of the types of radioactive decay (see 32.1), which consists in the capture of an electron from the inner layer by the nucleus.
31.3. INTERACTION OF X-RAY RADIATION WITH SUBSTANCE
The registration and use of X-ray radiation, as well as its impact on biological objects, are determined by the primary processes of interaction of an X-ray photon with electrons of atoms and molecules of a substance.
Depending on the ratio of energy hv photon and ionization energy 1 A and there are three main processes.
Coherent (classical) scattering
Scattering of long-wavelength X-rays occurs mainly without a change in wavelength, and is called coherent. It occurs if the photon energy is less than the ionization energy: hv< A and.
Since in this case the energy of the X-ray photon and the atom does not change, coherent scattering in itself does not cause a biological effect. However, when creating protection against X-ray radiation, one should take into account the possibility of changing the direction of the primary beam. This kind of interaction is important for X-ray diffraction analysis (see 24.7).
Incoherent scattering (Compton effect)
In 1922 A.Kh. Compton, observing the scattering of hard X-rays, discovered a decrease in the penetrating power of the scattered beam compared to the incident beam. This meant that the wavelength of the scattered X-rays was greater than that of the incident X-rays. The scattering of X-rays with a change in wavelength is called incoherent nym, and the phenomenon itself - the Compton effect. It occurs if the energy of the X-ray photon is greater than the ionization energy: hv > A and.
This phenomenon is due to the fact that when interacting with an atom, the energy hv photon is spent on the production of a new scattered X-ray photon with energy hv", to detach an electron from an atom (ionization energy A u) and impart kinetic energy to the electron E to:
hv \u003d hv " + A and + E k.(31.6)
1 Here, ionization energy is understood as the energy required to remove internal electrons from an atom or molecule.
Since in many cases hv>> A and and the Compton effect occurs on free electrons, then we can write approximately:
hv = hv"+ E K .(31.7)
It is significant that in this phenomenon (Fig. 31.9), along with secondary X-ray radiation (energy hv" photon) recoil electrons appear (kinetic energy E to electron). Atoms or molecules then become ions.
photoelectric effect
In the photoelectric effect, X-ray radiation is absorbed by an atom, as a result of which an electron flies out, and the atom is ionized (photoionization).
The three main interaction processes discussed above are primary, they lead to subsequent secondary, tertiary, etc. phenomena. For example, ionized atoms can emit a characteristic spectrum, excited atoms can become sources of visible light (X-ray luminescence), etc.
On fig. 31.10 is a diagram of the possible processes that occur when X-ray radiation enters a substance. Several tens of processes similar to the one shown may occur before the energy of the X-ray photon is converted into the energy of molecular thermal motion. As a result, there will be changes in the molecular composition of the substance.
The processes represented by the diagram in fig. 31.10, underlie the phenomena observed under the action of X-rays on matter. Let's list some of them.
X-ray luminescence- the glow of a number of substances under x-ray irradiation. Such a glow of platinum-cyanogen barium allowed Roentgen to discover the rays. This phenomenon is used to create special luminous screens for the purpose of visual observation of x-rays, sometimes to enhance the action of x-rays on a photographic plate.
The chemical action of X-ray radiation is known, for example, the formation of hydrogen peroxide in water. A practically important example is the effect on a photographic plate, which makes it possible to detect such rays.
The ionizing effect is manifested in an increase in electrical conductivity under the influence of X-rays. This property is used


in dosimetry to quantify the effect of this type of radiation.
As a result of many processes, the primary X-ray beam is weakened in accordance with the law (29.3). Let's write it in the form:
I = I0 e-/", (31.8)
Where μ - linear attenuation coefficient. It can be represented as consisting of three terms corresponding to coherent scattering μ κ , incoherent μ ΗΚ and photoeffect μ f:
μ = μ k + μ hk + μ f. (31.9)
The intensity of X-ray radiation is attenuated in proportion to the number of atoms of the substance through which this flow passes. If we compress matter along the axis x, for example, in b times by increasing b times its density, then

31.4. PHYSICAL FOUNDATIONS OF THE APPLICATION OF X-RAY RADIATION IN MEDICINE
One of the most important medical applications of X-rays is the transillumination of internal organs for diagnostic purposes. (X-ray diagnostics).
For diagnostics, photons with an energy of about 60-120 keV are used. At this energy, the mass extinction coefficient is mainly determined by the photoelectric effect. Its value is inversely proportional to the third power of the photon energy (proportional to λ 3), which manifests a large penetrating power of hard radiation, and proportional to the third power of the atomic number of the absorbing substance:

A significant difference in the absorption of x-ray radiation by different tissues allows you to see images of the internal organs of the human body in a shadow projection.
X-ray diagnostics is used in two versions: fluoroscopy the image is viewed on an X-ray luminescent screen, radiography - the image is fixed on the film.
If the organ under study and the surrounding tissues attenuate x-rays approximately equally, then special contrast agents are used. So, for example, filling the stomach and intestines with a mushy mass of barium sulfate, one can see their shadow image.
The brightness of the image on the screen and the exposure time on the film depend on the intensity of the x-rays. If it is used for diagnosis, then the intensity cannot be high, so as not to cause undesirable biological consequences. Therefore, there are a number of technical devices that improve the image at low X-ray intensities. An example of such a device is intensifier tubes (see 27.8). In a mass examination of the population, a variant of radiography is widely used - fluorography, in which an image from a large X-ray luminescent screen is recorded on a sensitive small-format film. When shooting, a lens of large aperture is used, the finished pictures are examined on a special magnifier.

An interesting and promising option for radiography is a method called x-ray tomography, and its "machine version" - CT scan.
Let's consider this question.
A plain radiograph covers a large area of the body, with various organs and tissues shading each other. You can avoid this if you periodically move the X-ray tube together (Fig. 31.11) in antiphase RT and film Fp relative to the object About research. The body contains a number of inclusions that are opaque to X-rays; they are shown by circles in the figure. As you can see, x-rays at any position of the x-ray tube (1, 2 etc.) pass through
cutting the same point of the object, which is the center, relative to which the periodic movement is performed RT And Fp. This point, more precisely a small opaque inclusion, is shown by a dark circle. His shadow image moves with fp, occupying successively positions 1, 2 etc. The remaining inclusions in the body (bones, seals, etc.) create on Fp some general background, since x-rays are not permanently obscured by them. By changing the position of the swing center, it is possible to obtain a layer-by-layer X-ray image of the body. Hence the name - tomography(layered recording).
It is possible, using a thin X-ray beam, to screen (instead of Fp), consisting of semiconductor detectors of ionizing radiation (see 32.5), and a computer, to process the shadow x-ray image in tomography. This modern version of tomography (computed or computed x-ray tomography) allows you to get layered images of the body on the screen of a cathode ray tube or on paper with details of less than 2 mm with a difference in x-ray absorption of up to 0.1%. This allows, for example, to distinguish between the gray and white matter of the brain and to see very small tumor formations.
FEDERAL AGENCY FOR EDUCATION OF THE RUSSIAN FEDERATION
STATE EDUCATIONAL INSTITUTION
HIGHER PROFESSIONAL EDUCATION
MOSCOW STATE INSTITUTE OF STEEL AND ALLOYS
(UNIVERSITY OF TECHNOLOGY)
NOVOTROITSKY BRANCH
Department of OEND
COURSE WORK
Discipline: Physics
Topic: X-RAY
Student: Nedorezova N.A.
Group: EiU-2004-25, No. З.К.: 04Н036
Checked by: Ozhegova S.M.
Introduction
Chapter 1
1.1 Biography of Roentgen Wilhelm Conrad
1.2 Discovery of X-rays
Chapter 2
2.1 X-ray sources
2.2 Properties of X-rays
2.3 Registration of X-rays
2.4 Use of X-rays
Chapter 3
3.1 Analysis of crystal structure imperfections
3.2 Spectrum analysis
Conclusion
List of sources used
Applications
Introduction
A rare person has not gone through an x-ray room. Pictures taken in x-rays are familiar to everyone. In 1995, this discovery was 100 years old. It is hard to imagine what great interest it aroused a century ago. In the hands of a man turned out to be an apparatus with which it was possible to see the invisible.
This invisible radiation, capable of penetrating, albeit to varying degrees, into all substances, which is electromagnetic radiation with a wavelength of about 10 -8 cm, was called X-ray radiation, in honor of Wilhelm Roentgen, who discovered it.
Like visible light, X-rays cause blackening of photographic film. This property is of great importance for medicine, industry and scientific research. Passing through the object under study and then falling on the film, X-ray radiation depicts its internal structure on it. Since the penetrating power of X-ray radiation is different for different materials, parts of the object that are less transparent to it give brighter areas in the photograph than those through which the radiation penetrates well. Thus, bone tissues are less transparent to x-rays than the tissues that make up the skin and internal organs. Therefore, on the radiograph, the bones will be indicated as lighter areas and the fracture site, which is less transparent for radiation, can be quite easily detected. X-ray imaging is also used in dentistry to detect caries and abscesses in the roots of teeth, as well as in industry to detect cracks in castings, plastics and rubbers, in chemistry to analyze compounds, and in physics to study the structure of crystals.
Roentgen's discovery was followed by experiments by other researchers who discovered many new properties and possibilities for using this radiation. A major contribution was made by M. Laue, W. Friedrich, and P. Knipping, who in 1912 demonstrated the diffraction of X-rays as they pass through a crystal; W. Coolidge, who in 1913 invented a high-vacuum X-ray tube with a heated cathode; G. Moseley, who established in 1913 the relationship between the wavelength of radiation and the atomic number of an element; G. and L. Braggi, who received the Nobel Prize in 1915 for developing the fundamentals of X-ray diffraction analysis.
The purpose of this course work is to study the phenomenon of x-ray radiation, the history of discovery, properties and identify the scope of its application.
Chapter 1
1.1 Biography of Roentgen Wilhelm Conrad
Wilhelm Conrad Roentgen was born on March 17, 1845 in the border region of Germany with Holland, in the city of Lenepe. He received his technical education in Zurich at the same Higher Technical School (Polytechnic) where Einstein later studied. Passion for physics forced him after leaving school in 1866 to continue physical education.
In 1868 he defended his dissertation for the degree of Doctor of Philosophy, he worked as an assistant at the Department of Physics, first in Zurich, then in Giessen, and then in Strasbourg (1874-1879) with Kundt. Here Roentgen went through a good experimental school and became a first-class experimenter. Roentgen performed part of the important research with his student, one of the founders of Soviet physics, A.F. Ioffe.
Scientific research relates to electromagnetism, crystal physics, optics, molecular physics.
In 1895, he discovered radiation with a wavelength shorter than the wavelength of ultraviolet rays (X-rays), later called x-rays, and investigated their properties: the ability to reflect, absorb, ionize air, etc. He proposed the correct design of the tube for obtaining X-rays - an inclined platinum anticathode and a concave cathode: he was the first to take photographs using X-rays. He discovered in 1885 the magnetic field of a dielectric moving in an electric field (the so-called "roentgen current"). His experience clearly showed that the magnetic field is created by moving charges, and was important for the creation of X. Lorentz's electronic theory. A significant number of Roentgen's works are devoted to the study properties of liquids, gases, crystals, electromagnetic phenomena, discovered the relationship between electrical and optical phenomena in crystals.For the discovery of the rays that bear his name, Roentgen in 1901 was the first among physicists to be awarded the Nobel Prize.
From 1900 until the last days of his life (he died on February 10, 1923) he worked at the University of Munich.
1.2 Discovery of X-rays
End of the 19th century was marked by increased interest in the phenomena of the passage of electricity through gases. Even Faraday seriously studied these phenomena, described various forms of discharge, discovered a dark space in a luminous column of rarefied gas. Faraday dark space separates the bluish, cathode glow from the pinkish, anode glow.
A further increase in the rarefaction of the gas significantly changes the nature of the glow. The mathematician Plücker (1801-1868) discovered in 1859, at sufficiently strong rarefaction, a weakly bluish beam of rays emanating from the cathode, reaching the anode and causing the glass of the tube to glow. Plücker's student Gittorf (1824-1914) in 1869 continued his teacher's research and showed that a distinct shadow appears on the fluorescent surface of the tube if a solid body is placed between the cathode and this surface.
Goldstein (1850-1931), studying the properties of rays, called them cathode rays (1876). Three years later, William Crookes (1832-1919) proved the material nature of cathode rays and called them "radiant matter" - a substance in a special fourth state. His evidence was convincing and clear. Experiments with the "Crookes tube" were demonstrated later in all physical classrooms . The deflection of the cathode beam by a magnetic field in a Crookes tube has become a classic school demonstration.
However, experiments on the electrical deflection of cathode rays were not so convincing. Hertz did not detect such a deviation and came to the conclusion that the cathode ray is an oscillatory process in the ether. Hertz's student F. Lenard, experimenting with cathode rays, showed in 1893 that they pass through a window covered with aluminum foil and cause a glow in the space behind the window. Hertz devoted his last article, published in 1892, to the phenomenon of the passage of cathode rays through thin metal bodies. It began with the words:
"Cathode rays differ from light in a significant way in terms of their ability to penetrate solids." Describing the results of experiments on the passage of cathode rays through gold, silver, platinum, aluminum, etc. leaves, Hertz notes that he did not observe any special differences in the phenomena The rays do not pass through the leaves in a straight line, but are scattered by diffraction.The nature of the cathode rays was still unclear.
It was with such tubes of Crookes, Lenard and others that the Würzburg professor Wilhelm Konrad Roentgen experimented at the end of 1895. Once, after the end of the experiment, he closed the tube with a black cardboard cover, turned off the light, but did not turn off the inductor that fed the tube, he noticed a glow of the screen from barium cyanogen located near the tube. Struck by this circumstance, Roentgen began to experiment with the screen. In his first report "On a new kind of rays", dated December 28, 1895, he wrote about these first experiments: "A piece of paper coated with barium platinum-cyanide, when approaching a tube, closed with a thin black cardboard cover that fits snugly enough to it, with each discharge it flashes with a bright light: it begins to fluoresce. Fluorescence is visible with sufficient darkening and does not depend on whether we bring the paper with the side coated with barium synerogen or not coated with barium synerogen. The fluorescence is noticeable even at a distance of two meters from the tube.”
Careful examination showed Roentgen "that black cardboard, transparent neither to the visible and ultraviolet rays of the sun, nor to the rays of an electric arc, is permeated with some kind of fluorescent agent." Roentgen investigated the penetrating power of this "agent", which he called for brevity "X-rays", for various substances. He found that the rays freely pass through paper, wood, ebonite, thin layers of metal, but are strongly delayed by lead.
He then describes the sensational experience:
“If you hold your hand between the discharge tube and the screen, you can see the dark shadows of the bones in the faint outlines of the shadow of the hand itself.” This was the first X-ray examination of the human body.
These shots made a huge impression; the discovery had not yet been completed, and X-ray diagnostics had already begun its journey. “My laboratory was flooded with doctors bringing in patients who suspected that they had needles in various parts of the body,” wrote the English physicist Schuster.
Already after the first experiments, Roentgen firmly established that X-rays differ from cathode ones, they do not carry a charge and are not deflected by a magnetic field, but they are excited by cathode rays. "X-rays are not identical with cathode rays, but they are excited by them in the glass walls of the discharge tube ”, wrote Roentgen.
He also established that they are excited not only in glass, but also in metals.
Mentioning the Hertz-Lenard hypothesis that cathode rays “are a phenomenon occurring in the ether,” Roentgen points out that “we can say something similar about our rays.” However, he failed to detect the wave properties of the rays, they "behave differently than hitherto known ultraviolet, visible, infrared rays." In their chemical and luminescent actions, they, according to Roentgen, are similar to ultraviolet rays. In the first message, he expressed the assumption left later that they can be longitudinal waves in the ether.
Roentgen's discovery aroused great interest in the scientific world. His experiments were repeated in almost all laboratories in the world. In Moscow they were repeated by P.N. Lebedev. In St. Petersburg, the inventor of radio A.S. Popov experimented with X-rays, demonstrated them at public lectures, receiving various X-rays. In Cambridge D.D. Thomson immediately applied the ionizing effect of X-rays to study the passage of electricity through gases. His research led to the discovery of the electron.
Chapter 2
X-ray radiation - electromagnetic ionizing radiation, occupying the spectral region between gamma and ultraviolet radiation within wavelengths from 10 -4 to 10 3 (from 10 -12 to 10 -5 cm).R. l. with wavelength λ< 2 условно называются жёсткими, с λ >2 - soft.
2.1 X-ray sources
The most common source of X-rays is the X-ray tube.
X-ray tubes are used in X-ray structural analysis
The main characteristics of X-ray tubes are the maximum permissible accelerating voltage (1-500 kV), electronic current (0.01 mA - 1A), specific power dissipated by the anode (10-10 4 W / mm 2), total power consumption (0.002 W - 60 kW) and focus sizes (1 µm - 10 mm). The efficiency of the x-ray tube is 0.1-3%.
Some radioactive isotopes can also serve as sources of X-rays.
Synchrotrons and electron storage rings with energies of several GeV can serve as sources of soft X-rays with λ on the order of tens and hundreds. In intensity, the X-ray radiation of synchrotrons exceeds the radiation of an X-ray tube in the specified region of the spectrum by 2-3 orders of magnitude.
Natural sources of X-rays - the Sun and other space objects.
2.2 Properties of X-rays
Depending on the mechanism of origin of X-rays, their spectra can be continuous (bremsstrahlung) or line (characteristic). A continuous X-ray spectrum is emitted by fast charged particles as a result of their deceleration when interacting with target atoms; this spectrum reaches a significant intensity only when the target is bombarded with electrons. The intensity of bremsstrahlung X-rays is distributed over all frequencies up to the high-frequency boundary 0 , at which the photon energy h 0 (h is Planck's constant
Line radiation occurs after the ionization of an atom with the ejection of an electron from one of its inner shells. Such ionization can be the result of an atom colliding with a fast particle, such as an electron (primary x-rays), or the absorption of a photon by an atom (fluorescent x-rays). The ionized atom finds itself in the initial quantum state at one of the high energy levels and after 10 -16 -10 -15 seconds passes into the final state with a lower energy. In this case, an atom can emit an excess of energy in the form of a photon of a certain frequency. The frequencies of the lines of the spectrum of such radiation are characteristic of the atoms of each element, therefore the line X-ray spectrum is called characteristic. The dependence of the line frequency of this spectrum on the atomic number Z is determined by the Moseley law.
Moseley's law, the law relating the frequency of the spectral lines of the characteristic X-ray emission of a chemical element with its serial number. G. Moseley experimentally installed
where R is the Rydberg constant
Moseley's law was irrefutable proof of the correct placement of elements in the periodic table of elements
In accordance with Moseley's law, X-ray characteristic spectra do not exhibit the periodic patterns inherent in optical spectra. This indicates that the inner electron shells of atoms of all elements that appear in the characteristic X-ray spectra have a similar structure.
Later experiments revealed some deviations from the linear dependence for the transition groups of elements, associated with a change in the order of filling of the outer electron shells, as well as for heavy atoms, appearing as a result of relativistic effects (conditionally explained by the fact that the speeds of the inner ones are comparable to the speed of light).
Depending on a number of factors - on the number of nucleons in the nucleus (isotonic shift), the state of the outer electron shells (chemical shift), etc. - the position of the spectral lines on the Moseley diagram may change somewhat. The study of these shifts allows one to obtain detailed information about the atom.
Bremsstrahlung X-rays emitted by very thin targets are completely polarized near 0; as 0 decreases, the degree of polarization decreases. Characteristic radiation, as a rule, is not polarized.
When X-rays interact with matter, the photoelectric effect can occur.
When X-rays pass through a layer of substance with thickness x, their initial intensity I 0 decreases to the value I = I 0 e - μ x where μ is the attenuation coefficient. The attenuation of I occurs due to two processes: the absorption of X-ray photons by matter and the change in their direction upon scattering. In the long-wavelength region of the spectrum, the absorption of X-rays predominates, in the short-wavelength region, their scattering. The degree of absorption increases rapidly with increasing Z and λ. For example, hard X-rays freely penetrate through a layer of air ~ 10 cm; an aluminum plate 3 cm thick attenuates X-rays with λ = 0.027 by half; soft x-rays are significantly absorbed in air and their use and study is possible only in a vacuum or in a weakly absorbing gas (for example, He). When X-rays are absorbed, the atoms of a substance are ionized.
The effect of X-rays on living organisms can be beneficial or harmful, depending on the ionization they cause in the tissues. Since the absorption of X-rays depends on λ, their intensity cannot serve as a measure of the biological effect of X-rays. X-ray measurements are used to measure the effect of X-rays on matter.
Scattering of X-rays in the region of large Z and λ occurs mainly without a change in λ and is called coherent scattering, while in the region of small Z and λ, as a rule, it increases (incoherent scattering). There are 2 types of incoherent X-ray scattering - Compton and Raman. In Compton scattering, which has the character of inelastic corpuscular scattering, a recoil electron flies out of the atomic shell due to the energy partially lost by the X-ray photon. In this case, the energy of the photon decreases and its direction changes; the change in λ depends on the scattering angle. During Raman scattering of a high-energy X-ray photon by a light atom, a small part of its energy is spent on ionization of the atom and the direction of the photon's motion changes. The change of such photons does not depend on the scattering angle.
The refractive index n for x-rays differs from 1 by a very small amount δ = 1-n ≈ 10 -6 -10 -5 . The phase velocity of X-rays in a medium is greater than the speed of light in a vacuum. The deviation of X-rays during the transition from one medium to another is very small (a few arc minutes). When X-rays fall from a vacuum onto the surface of a body at a very small angle, their total external reflection occurs.
2.3 Registration of X-rays
The human eye is not sensitive to x-rays. X-ray
rays are recorded using a special x-ray film containing an increased amount of Ag, Br. In the region λ<0,5 чувствительность этих плёнок быстро падает и может быть
искусственно повышена плотно прижатым к плёнке флуоресцирующим экраном. В
области λ>5, the sensitivity of ordinary positive film is quite high, and its grains are much smaller than the grains of X-ray film, which increases the resolution. At λ of the order of tens and hundreds, X-rays act only on the thinnest surface layer of the photographic emulsion; to increase the sensitivity of the film, it is sensitized with luminescent oils. In X-ray diagnostics and flaw detection, electrophotography is sometimes used to record X-rays.
X-rays of high intensity can be recorded using an ionization chamber
2.4 Use of X-rays
X-rays are most widely used in medicine for X-ray diagnostics.
X-ray structural analysis
X-ray microscopy
X-rays coming from space carry information about the chemical composition of cosmic bodies and about the physical processes taking place in space. X-ray astronomy deals with the study of cosmic x-rays
Chapter 3
One of the main tasks of X-ray diffraction analysis is the determination of the real or phase composition of a material. The X-ray diffraction method is direct and is characterized by high reliability, rapidity and relative cheapness. The method does not require a large amount of substance, the analysis can be carried out without destroying the part. The areas of application of qualitative phase analysis are very diverse both for scientific research and for control in production. You can check the composition of the raw materials of metallurgical production, synthesis products, processing, the result of phase changes during thermal and chemical-thermal treatment, analyze various coatings, thin films, etc.
Each phase, having its own crystal structure, is characterized by a certain set of discrete values of interplanar distances d/n from the maximum and below, inherent only to this phase. As follows from the Wulf-Bragg equation, each value of the interplanar distance corresponds to a line on the x-ray pattern from a polycrystalline sample at a certain angle θ (at a given value of the wavelength λ). Thus, a certain system of lines (diffraction maxima) will correspond to a certain set of interplanar distances for each phase in the X-ray diffraction pattern. The relative intensity of these lines in the X-ray pattern depends primarily on the structure of the phase. Therefore, by determining the location of the lines on the radiograph (its angle θ) and knowing the wavelength of the radiation at which the radiograph was taken, it is possible to determine the values of the interplanar distances d/n using the Wulf-Bragg formula:
/n = λ/ (2sin θ). (1)
Having determined the set of d/n for the material under study and comparing it with the previously known d/n data for pure substances, their various compounds, it is possible to establish which phase the given material comprises. It should be emphasized that it is the phases that are determined, and not the chemical composition, but the latter can sometimes be deduced if there are additional data on the elemental composition of a particular phase. The task of qualitative phase analysis is greatly facilitated if the chemical composition of the material under study is known, because then it is possible to make preliminary assumptions about the possible phases in this case.
The key to phase analysis is to accurately measure d/n and line intensity. Although this is in principle easier to achieve using a diffractometer, the photomethod for qualitative analysis has some advantages, primarily in terms of sensitivity (the ability to detect the presence of a small amount of phase in the sample), as well as the simplicity of the experimental technique.
The calculation of d/n from the X-ray pattern is carried out using the Wulf-Bragg equation.
As the value of λ in this equation, λ α cf K-series is usually used:
λ α cf = (2λ α1 + λ α2) /3 (2)
Sometimes the K α1 line is used. Determining the diffraction angles θ for all X-ray lines allows you to calculate d / n according to equation (1) and separate the β-lines (if there was no filter for (β-rays).
3.1 Analysis of crystal structure imperfections
All real single-crystal and even more so polycrystalline materials contain certain structural imperfections (point defects, dislocations, various types of interfaces, micro- and macrostresses), which have a very strong effect on all structure-sensitive properties and processes.
Structural imperfections cause distortions of the crystal lattice of different nature and, as a result, different types of changes in the diffraction pattern: a change in interatomic and interplanar distances causes a shift in diffraction maxima, microstresses and dispersity of the substructure lead to a broadening of diffraction maxima, lattice microdistortions - to a change in the intensity of these maxima, the presence dislocations causes anomalous phenomena during the passage of X-rays and, consequently, local contrast inhomogeneities on X-ray topograms, etc.
As a result, X-ray diffraction analysis is one of the most informative methods for studying structural imperfections, their type and concentration, and the nature of their distribution.
The traditional direct method of X-ray diffraction, which is implemented on stationary diffractometers, due to their design features, allows quantitative determination of stresses and strains only on small samples cut from parts or objects.
Therefore, at present, there is a transition from stationary to portable small-sized X-ray diffractometers, which provide an assessment of stresses in the material of parts or objects without destruction at the stages of their manufacture and operation.
Portable X-ray diffractometers of the DRP * 1 series make it possible to control residual and effective stresses in large-sized parts, products and structures without destruction
The program in the Windows environment allows not only to determine the stresses using the "sin 2 ψ" method in real time, but also to monitor the change in the phase composition and texture. The linear coordinate detector provides simultaneous registration at diffraction angles 2θ = 43°. small-sized X-ray tubes of the "Fox" type with high luminosity and low power (5 W) ensure the radiological safety of the device, in which at a distance of 25 cm from the irradiated area, the radiation level is equal to the natural background level. Devices of the DRP series are used in determining stresses at various stages of metal forming, cutting, grinding, heat treatment, welding, surface hardening in order to optimize these technological operations. Control over the drop in the level of induced residual compressive stresses in especially critical products and structures during their operation makes it possible to take the product out of service before its destruction, preventing possible accidents and catastrophes.
3.2 Spectrum analysis
Along with the determination of the atomic crystal structure and phase composition of the material, for its complete characterization, it is obligatory to determine its chemical composition.
Increasingly, various so-called instrumental methods of spectral analysis are used in practice for these purposes. Each of them has its own advantages and applications.
One of the important requirements in many cases is that the method used ensures the safety of the analyzed object; It is these methods of analysis that are discussed in this section. The next criterion according to which the methods of analysis described in this section were chosen is their locality.
The method of fluorescence X-ray spectral analysis is based on the penetration of rather hard X-ray radiation (from an X-ray tube) into the analyzed object, penetrating into a layer with a thickness of the order of several micrometers. The characteristic X-ray radiation arising in this case in the object makes it possible to obtain averaged data on its chemical composition.
To determine the elemental composition of a substance, one can use the analysis of the characteristic X-ray spectrum of a sample placed on the anode of an X-ray tube and subjected to electron bombardment - the emission method, or the analysis of the spectrum of secondary (fluorescent) X-ray radiation of a sample subjected to irradiation with hard X-rays from an X-ray tube or other source - fluorescent method.
The disadvantage of the emission method is, firstly, the need to place the sample on the anode of the X-ray tube, followed by evacuation with vacuum pumps; obviously, this method is unsuitable for fusible and volatile substances. The second drawback is related to the fact that even refractory objects are damaged by electron bombardment. The fluorescent method is free from these shortcomings and therefore has a much wider application. The advantage of the fluorescence method is also the absence of bremsstrahlung, which improves the sensitivity of the analysis. Comparison of the measured wavelengths with tables of spectral lines of chemical elements is the basis of a qualitative analysis, and the relative intensities of the spectral lines of different elements that form the sample substance form the basis of a quantitative analysis. From a consideration of the mechanism of excitation of characteristic X-ray radiation, it is clear that the radiations of one or another series (K or L, M, etc.) arise simultaneously, and the ratio of line intensities within the series is always constant. Therefore, the presence of this or that element is established not by individual lines, but by a series of lines as a whole (except for the weakest ones, taking into account the content of this element). For relatively light elements, the analysis of the K-series lines is used, for heavy elements, the L-series lines; under different conditions (depending on the equipment used and on the analyzed elements), different regions of the characteristic spectrum may be most convenient.
The main features of X-ray spectral analysis are as follows.
Simplicity of X-ray characteristic spectra even for heavy elements (compared to optical spectra), which simplifies the analysis (small number of lines; similarity in their mutual arrangement; with an increase in the serial number, a regular shift of the spectrum to the short-wavelength region occurs; comparative simplicity of quantitative analysis).
Independence of wavelengths from the state of atoms of the analyzed element (free or in a chemical compound). This is due to the fact that the occurrence of characteristic X-ray radiation is associated with the excitation of internal electronic levels, which in most cases practically do not change with the degree of ionization of atoms.
The possibility of separation in the analysis of rare earth and some other elements that have small differences in the spectra in the optical range due to the similarity of the electronic structure of the outer shells and differ very little in their chemical properties.
X-ray fluorescence spectroscopy is "non-destructive", so it has an advantage over conventional optical spectroscopy when analyzing thin samples - thin metal sheet, foil, etc.
X-ray fluorescence spectrometers, among them multichannel spectrometers or quantometers, providing express quantitative analysis of elements (from Na or Mg to U) with an error of less than 1% of the determined value, a sensitivity threshold of 10 -3 ... 10 -4% .
x-ray beam
Methods for determining the spectral composition of x-rays
Spectrometers are divided into two types: crystal-diffraction and crystalless.
The decomposition of X-rays into a spectrum using a natural diffraction grating - a crystal - is essentially similar to obtaining a spectrum of ordinary light rays using an artificial diffraction grating in the form of periodic strokes on glass. The condition for the formation of a diffraction maximum can be written as the condition of "reflection" from a system of parallel atomic planes separated by a distance d hkl .
When conducting a qualitative analysis, one can judge the presence of an element in a sample by one line - usually the most intense line of the spectral series suitable for a given analyzer crystal. The resolution of crystal diffraction spectrometers is sufficient to separate the characteristic lines even of elements adjacent in position in the periodic table. However, it is also necessary to take into account the imposition of different lines of different elements, as well as the imposition of reflections of different orders. This circumstance should be taken into account when choosing analytical lines. At the same time, it is necessary to use the possibilities of improving the resolution of the device.
Conclusion
Thus, x-rays are invisible electromagnetic radiation with a wavelength of 10 5 - 10 2 nm. X-rays can penetrate some materials that are opaque to visible light. They are emitted during the deceleration of fast electrons in matter (continuous spectrum) and during transitions of electrons from the outer electron shells of the atom to the inner ones (linear spectrum). Sources of X-ray radiation are: X-ray tube, some radioactive isotopes, accelerators and accumulators of electrons (synchrotron radiation). Receivers - film, luminescent screens, nuclear radiation detectors. X-rays are used in X-ray diffraction analysis, medicine, flaw detection, X-ray spectral analysis, etc.
Having considered the positive aspects of V. Roentgen's discovery, it is necessary to note its harmful biological effect. It turned out that X-rays can cause something like a severe sunburn (erythema), accompanied, however, by deeper and more permanent damage to the skin. Appearing ulcers often turn into cancer. In many cases, fingers or hands had to be amputated. There were also deaths.
It has been found that skin damage can be avoided by reducing exposure time and dose, using shielding (eg lead) and remote controls. But gradually other, more long-term effects of X-ray exposure were revealed, which were then confirmed and studied in experimental animals. Effects due to X-rays and other ionizing radiations (such as gamma rays emitted by radioactive materials) include:
) temporary changes in the composition of the blood after a relatively small excess exposure;
) irreversible changes in the composition of the blood (hemolytic anemia) after prolonged excessive exposure;
) an increase in the incidence of cancer (including leukemia);
) faster aging and early death;
) the occurrence of cataracts.
The biological impact of X-rays on the human body is determined by the level of radiation dose, as well as by which particular organ of the body was exposed to radiation.
The accumulation of knowledge about the effects of X-ray radiation on the human body has led to the development of national and international standards for permissible radiation doses, published in various reference books.
To avoid the harmful effects of X-rays, control methods are used:
) availability of adequate equipment,
) monitoring compliance with safety regulations,
) correct use of the equipment.
List of sources used
1) Blokhin M.A., Physics of X-rays, 2nd ed., M., 1957;
) Blokhin M.A., Methods of X-ray spectral studies, M., 1959;
) X-rays. Sat. ed. M.A. Blokhin, trans. with him. and English, M., 1960;
) Kharaja F., General course of X-ray engineering, 3rd ed., M. - L., 1966;
) Mirkin L.I., Handbook of X-ray diffraction analysis of polycrystals, M., 1961;
) Weinstein E.E., Kakhana M.M., Reference tables on X-ray spectroscopy, M., 1953.
) X-ray and electron-optical analysis. Gorelik S.S., Skakov Yu.A., Rastorguev L.N.: Proc. Allowance for universities. - 4th ed. Add. And a reworker. - M.: "MISiS", 2002. - 360 p.
Applications
Annex 1
General view of X-ray tubes

Annex 2
Scheme of X-ray tube for structural analysis

Scheme of an X-ray tube for structural analysis: 1 - metal anode glass (usually grounded); 2 - windows made of beryllium for x-ray output; 3 - thermionic cathode; 4 - glass bulb, isolating the anode part of the tube from the cathode; 5 - cathode terminals, to which the filament voltage is applied, as well as high (relative to the anode) voltage; 6 - electrostatic system for focusing electrons; 7 - anode (anticathode); 8 - branch pipes for input and output of running water cooling the anode glass.
Annex 3
Moseley diagram

Moseley diagram for K-, L- and M-series of characteristic X-rays. The abscissa shows the serial number of the element Z, the ordinate - ( With is the speed of light).
Appendix 4
Ionization chamber.

Fig.1. Section of a cylindrical ionization chamber: 1 - cylindrical body of the chamber, which serves as a negative electrode; 2 - cylindrical rod serving as a positive electrode; 3 - insulators.

Rice. 2. Scheme of switching on the current ionization chamber: V - voltage on the electrodes of the chamber; G is a galvanometer that measures the ionization current.

Rice. 3. Current-voltage characteristic of the ionization chamber.

Rice. 4. Scheme of switching on the pulsed ionization chamber: C - capacitance of the collecting electrode; R is resistance.
Annex 5
Scintillation counter.

Scheme of a scintillation counter: light quanta (photons) "knock out" electrons from the photocathode; moving from dynode to dynode, the electron avalanche multiplies.
Appendix 6
Geiger-Muller counter.

Rice. 1. Scheme of a glass Geiger-Muller counter: 1 - hermetically sealed glass tube; 2 - cathode (a thin layer of copper on a stainless steel tube); 3 - output of the cathode; 4 - anode (thin stretched thread).

Rice. 2. Scheme of switching on the Geiger-Muller counter.

Rice. 3. The counting characteristic of the Geiger-Muller counter.
Annex 7
proportional counter.

Scheme of a proportional counter: a - electron drift region; b - area of gas amplification.
Appendix 8
Semiconductor detectors
Semiconductor detectors; the sensitive area is highlighted by hatching; n - region of a semiconductor with electronic conductivity, p - with hole, i - with intrinsic conduction; a - silicon surface-barrier detector; b - drift germanium-lithium planar detector; c - germanium-lithium coaxial detector.



